How to make aquarium water harder is a question that often arises for fish keepers, especially those who are new to the hobby. Many freshwater fish species, particularly those originating from tropical and subtropical regions, thrive in hard water environments.
These fish rely on the presence of minerals like calcium and magnesium for proper bone development, shell formation, and overall health. While tap water can sometimes provide adequate hardness levels, understanding how to adjust your aquarium’s water hardness is essential for ensuring the well-being of your fish.
This guide will delve into the importance of water hardness for specific fish species, exploring the different methods available for increasing water hardness, and outlining the key steps involved in maintaining a stable and healthy hard water environment for your aquarium.
Understanding Water Hardness

Water hardness is a crucial factor in maintaining a healthy aquarium environment. It refers to the concentration of dissolved minerals, primarily calcium and magnesium, in the water. These minerals play a vital role in various biological processes, including shell formation, bone growth, and overall fish health.
Understanding water hardness is essential for aquarists to ensure their fish thrive in a suitable environment.
Increasing the hardness of your aquarium water can be achieved through various methods, such as adding calcium and magnesium salts. However, maintaining a stable temperature is crucial for fish health, and this is where understanding where to place the aquarium heater becomes vital.
Proper heater placement ensures even temperature distribution, minimizing fluctuations that can impact water hardness levels and overall aquarium stability.
Types of Water Hardness
Water hardness can be categorized into two main types: general hardness (GH) and carbonate hardness (KH).
- General Hardness (GH): This measures the total concentration of calcium and magnesium ions in the water. It is a crucial factor in determining the overall mineral content and its impact on fish health.
- Carbonate Hardness (KH): This measures the concentration of carbonate and bicarbonate ions in the water. These ions act as a buffer, helping to maintain a stable pH level, which is essential for fish survival.
Units of Measurement
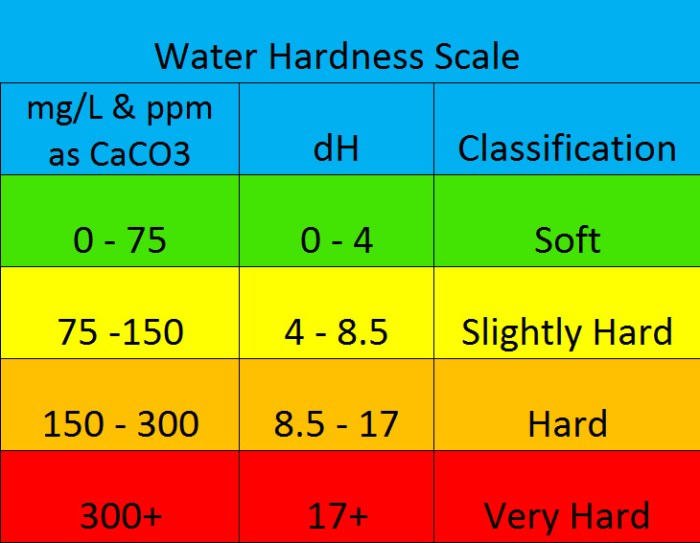
Water hardness is typically measured in parts per million (ppm), degrees of German hardness (dGH), and degrees of KH (dKH).
- Parts Per Million (ppm): This unit represents the number of milligrams of dissolved minerals per liter of water.
- Degrees of German Hardness (dGH): This unit is equivalent to 17.8 ppm of calcium carbonate (CaCO 3). A dGH of 10 represents a concentration of 178 ppm of CaCO 3.
- Degrees of KH (dKH): This unit is also equivalent to 17.8 ppm of CaCO 3, but it specifically measures the concentration of carbonate and bicarbonate ions.
For example, a water sample with a GH of 10 dGH and a KH of 5 dKH indicates that it contains 178 ppm of calcium and magnesium ions (total hardness) and 89 ppm of carbonate and bicarbonate ions (carbonate hardness).
Increasing the hardness of your aquarium water can be achieved through various methods, including adding calcium and magnesium supplements. For those looking to add a decorative touch to their tank, consider incorporating aquarium safe sculpting materials like ceramic or resin, as these materials won’t leach harmful chemicals into the water and can help maintain the desired hardness levels.
However, always ensure that any materials added to your tank are thoroughly researched for their compatibility with your fish and aquatic environment.
Why Hard Water is Necessary for Some Fish
Not all fish species thrive in the same water conditions. While some prefer soft water, others require hard water to flourish. This is due to the specific needs of their physiology and the role of calcium in their well-being.Hard water, rich in minerals like calcium and magnesium, provides essential benefits for certain fish species.
These benefits extend beyond just basic survival and contribute to their overall health and reproductive success.
Calcium Absorption and Shell Formation, How to make aquarium water harder
Calcium is a vital mineral for many fish, particularly those with hard shells or bony structures. Fish like African cichlids, snails, and shrimp require sufficient calcium in their environment for proper shell development and maintenance.
- African cichlids, renowned for their vibrant colors and diverse species, rely heavily on calcium for their strong, hard scales and bony structures. Hard water provides a readily available source of calcium, enabling these fish to develop and maintain their unique features.
In soft water, cichlids may experience stunted growth, weak scales, and difficulty forming eggs, impacting their reproductive success.
- Snails, such as the popular mystery snail, utilize calcium to build their shells. Adequate calcium levels in hard water ensure their shells grow strong and resilient, protecting them from predators and environmental stress. In soft water, snails may struggle to form their shells properly, leading to thin, fragile shells and an increased risk of injury.
- Shrimp, particularly freshwater shrimp like cherry shrimp, require calcium for their exoskeletons. Hard water provides the necessary calcium for their exoskeletons to grow and molt properly. In soft water, shrimp may experience stunted growth, weak exoskeletons, and difficulties molting, leaving them vulnerable to predators and disease.
Methods for Increasing Water Hardness
Increasing the hardness of your aquarium water is crucial for certain fish species that thrive in harder water conditions. This is achieved by adding minerals like calcium and magnesium to the water. There are several methods available to increase water hardness, each with its own advantages and disadvantages.
Adding Calcium Chloride or Calcium Carbonate
Adding calcium chloride (CaCl2) or calcium carbonate (CaCO3) directly to your aquarium water is a straightforward method for increasing water hardness. These chemicals readily dissolve in water, increasing the concentration of calcium ions, which directly contributes to hardness.Calcium chloride is more soluble than calcium carbonate, making it more efficient for raising hardness quickly.
However, it can also lower the pH of your aquarium water, which may not be suitable for all fish species. Calcium carbonate, on the other hand, is more pH-neutral but dissolves more slowly, requiring a longer time to achieve the desired hardness level.
It’s important to note that adding too much calcium chloride or calcium carbonate can lead to an abrupt change in water parameters, potentially stressing your fish. It’s crucial to add these chemicals gradually and monitor the water hardness levels closely.
Using Crushed Coral or Seashells
Crushed coral or seashells are natural sources of calcium carbonate. These materials are added to the aquarium substrate or filter media, gradually releasing calcium ions into the water as they dissolve. This method provides a slow and steady increase in water hardness, mimicking the natural environment of many reef fish.The use of crushed coral or seashells is a more natural and less intrusive approach than directly adding chemicals.
However, it takes a longer time to achieve the desired hardness level, and the rate of dissolution can vary depending on the size and type of material used.
It’s important to choose crushed coral or seashells that are specifically designed for aquariums and free from harmful contaminants.
Employing a Dedicated Water Hardness Increaser
Dedicated water hardness increasers are commercially available products designed to increase water hardness safely and effectively. These products typically contain a blend of calcium and magnesium salts, along with other additives that may help buffer the pH of the water.These products are generally easy to use and provide a controlled and predictable way to increase water hardness.
However, they can be more expensive than other methods and may contain additional chemicals that could potentially affect the water quality if not used as directed.
Always read the product label carefully and follow the manufacturer’s instructions for use.
Comparison of Methods
| Method | Cost | Effectiveness | Potential Side Effects |
|---|---|---|---|
| Adding Calcium Chloride or Calcium Carbonate | Low | High | pH lowering (CaCl2), rapid changes in water parameters |
| Using Crushed Coral or Seashells | Moderate | Moderate | Slower rate of hardness increase |
| Employing a Dedicated Water Hardness Increaser | High | High | Potential for additional chemicals in the water |
Maintaining Hard Water Parameters

Maintaining the desired hardness levels in your aquarium is crucial for the well-being of your fish. Regular monitoring and adjustments are essential to ensure a stable and healthy environment.
Regular Water Testing
Regular water testing is essential for monitoring water hardness levels. This allows you to identify any fluctuations or deviations from the desired parameters and make necessary adjustments. Water hardness testing kits are readily available at most pet stores. These kits typically measure general hardness (GH) and carbonate hardness (KH), which are crucial indicators of the water’s overall hardness.
Regular water testing allows you to identify any fluctuations or deviations from the desired parameters and make necessary adjustments.
Adjusting Water Hardness Levels
Adjusting water hardness levels is necessary to ensure the optimal conditions for your specific fish species. Some fish thrive in hard water, while others require softer water. Here’s a breakdown of how to adjust water hardness levels based on your fish’s needs:
Increasing Water Hardness
- Adding Calcium and Magnesium Salts:The most common way to increase water hardness is by adding calcium and magnesium salts. These salts can be purchased in liquid or granular form at pet stores.
- Using Crushed Coral:Crushed coral is a natural source of calcium and magnesium. It can be added to the aquarium filter or directly to the tank.
- Adding Baking Soda:Baking soda (sodium bicarbonate) can be used to increase carbonate hardness (KH). However, it’s important to use it sparingly as excessive amounts can raise the pH levels.
Decreasing Water Hardness
- Using Reverse Osmosis (RO) Water:RO water is purified water that has been stripped of minerals, including those that contribute to water hardness. It can be used to dilute hard water, effectively lowering its hardness.
- Adding Peat Moss:Peat moss can help soften water by releasing humic acids, which can bind to calcium and magnesium ions.
- Using Water Softeners:Water softeners are devices that remove calcium and magnesium ions from water. They are typically used for household water softening but can also be used to soften aquarium water.
Risks of Over-Hardening Aquarium Water
While maintaining appropriate hardness levels is crucial, over-hardening the water can pose several risks to your fish.
- Stress and Illness:Over-hard water can stress fish, making them more susceptible to illness.
- Scale Rot:In extreme cases, over-hard water can contribute to scale rot, a bacterial infection that affects the fish’s scales.
- Reduced Oxygen Levels:Hard water can reduce the amount of dissolved oxygen in the water, making it difficult for fish to breathe.
Natural Sources of Hard Water: How To Make Aquarium Water Harder
Natural sources of hard water are often readily available and can be a cost-effective way to increase the hardness of your aquarium water. However, it’s crucial to understand the potential benefits and drawbacks of using these sources and to properly test and treat the water before introducing it to your fish.
Well Water
Well water is often naturally hard due to the presence of dissolved minerals like calcium and magnesium. These minerals are picked up as the water travels through the ground.
- Benefits:Well water is often a readily available and inexpensive source of hard water. It can also be a more sustainable option than using bottled water or other artificial hardeners.
- Drawbacks:Well water can contain other impurities besides minerals, such as bacteria, pesticides, or heavy metals. These contaminants can be harmful to fish and may require treatment before using the water in an aquarium.
Spring Water
Spring water is another natural source of hard water. It is often considered a more pure and cleaner source of water than well water, as it originates from underground springs.
- Benefits:Spring water is often naturally hard and free from contaminants. It is also often bottled and readily available, making it a convenient option for aquarists.
- Drawbacks:Bottled spring water can be expensive and may not be the most sustainable option. It’s also important to note that not all spring water is naturally hard. Some brands may be demineralized or treated to reduce hardness, so it’s essential to check the label.
Testing and Treating Natural Water Sources
Before using any natural water source in your aquarium, it’s crucial to test it for hardness and other parameters, such as pH, ammonia, nitrite, and nitrate.
- Hardness:Use a water hardness test kit to determine the GH and KH levels of the water. Adjust the hardness levels as needed using methods discussed previously.
- Other Parameters:Test for pH, ammonia, nitrite, and nitrate to ensure the water is safe for your fish. If necessary, treat the water to remove harmful contaminants using appropriate methods, such as dechlorination, filtration, or chemical treatment.
It’s always best to err on the side of caution and test your water thoroughly before using it in your aquarium. This will help ensure the health and well-being of your fish.
Hard Water Aquarium Setup
Creating a hard water aquarium setup requires careful consideration of the substrate, plants, and decorations to ensure a stable and healthy environment for your fish.
Substrate Selection
The substrate plays a crucial role in maintaining hard water parameters.
- Crushed Coral:Crushed coral is a popular choice for hard water aquariums, as it naturally raises the pH and GH. It also provides a visually appealing and porous substrate that benefits beneficial bacteria.
- Aragonite Sand:Aragonite sand is another effective option, as it is composed of calcium carbonate, which contributes to water hardness. It is available in various sizes and colors, offering aesthetic versatility.
- Limestone Gravel:Limestone gravel is a cost-effective alternative to crushed coral and aragonite sand. It is rich in calcium carbonate and readily raises water hardness.
Plant Selection
While hard water conditions can be challenging for some plants, there are several species that thrive in these environments.
- Java Fern:Java ferns are hardy and adaptable, tolerating a wide range of water parameters, including hard water.
- Anubias:Anubias species are known for their resilience and ability to thrive in hard water, even with low light conditions.
- Cryptocoryne:Cryptocoryne plants are popular for their unique leaf shapes and colors. Some species are particularly well-suited to hard water environments.
Decoration Selection
Decorations can enhance the aesthetic appeal of your aquarium and provide hiding places for your fish.
- Driftwood:Driftwood is a natural addition that provides a sense of realism and can also help soften the water slightly.
- Rocks:Limestone rocks are a suitable choice for hard water aquariums, as they contribute to water hardness and provide a natural look.
- Ceramic Ornaments:Ceramic ornaments are available in a variety of styles and can add visual interest to your aquarium.
Maintaining Hard Water Parameters
Maintaining stable hard water parameters is crucial for the health and well-being of your fish.
- Regular Water Changes:Regular water changes are essential to replenish minerals and remove accumulated waste, helping to maintain stable water hardness.
- Water Testing:Regularly testing your water parameters with a reliable test kit is vital to monitor GH and pH levels.
- Mineral Supplements:If necessary, you can use mineral supplements to adjust water hardness levels, but it’s important to do so gradually and monitor the effects closely.
Examples of Successful Hard Water Aquarium Setups
- African Cichlid Tank:A popular choice for hard water enthusiasts, African cichlid tanks typically feature a substrate of crushed coral or aragonite sand, along with limestone rocks and driftwood. Water parameters are maintained at a pH of 7.5-8.5 and a GH of 10-15 dGH.
Common fish species include Mbuna cichlids, such as Pseudotropheus zebra, and Peacocks, such as Aulonocara stuartgranti.
- Discus Tank:Discus fish are known for their sensitivity to water parameters, requiring hard water with a pH of 6.0-7.0 and a GH of 5-10 dGH. A discus tank setup might include a substrate of fine gravel or sand, along with driftwood and plants like Anubias and Java fern.
Summary
By understanding the concept of water hardness, identifying the needs of your fish, and employing appropriate methods to adjust the water parameters, you can create a thriving aquarium environment that supports the health and longevity of your aquatic companions. Remember, regular water testing is crucial for maintaining the desired water hardness levels, and adjusting your methods as needed ensures a stable and balanced ecosystem for your fish.